Abstract
Richter syndrome (RS), the development of diffuse large B-cell lymphoma (DLBCL) in the context of CLL, is an uncommon complication with a dismal prognosis. Currently, data on biological and clinical features, and factors predicting the outcome of patients are scarce, and there is still no consensus on the best therapeutic approach for these patients (pts). The aim of this study was to analyze the presenting characteristics, treatment outcomes, and factors predicting for survival in a series of pts with RS from a large population-based cohort of the Spanish CLL Study Group (GELLC).
Pts diagnosed with RS during 1988 - 2017 were retrospectively identified in eleven centers of the GELLC group. Only cases with confirmed biopsy-proven RS during follow-up were included. Clinical-biological features at diagnosis of RS, including cytogenetics, clonal relation with the pre-existing CLL, Epstein-Barr virus (EBV) status detected by in situ hybridization, cell of origin (COO) analyzed by immunohistochemistry, International Prognostic Index score (IPI) and the RS score (Tsimberidou AM et al, J Clin Oncol, 2006), as well as treatment and outcomes, were analyzed.
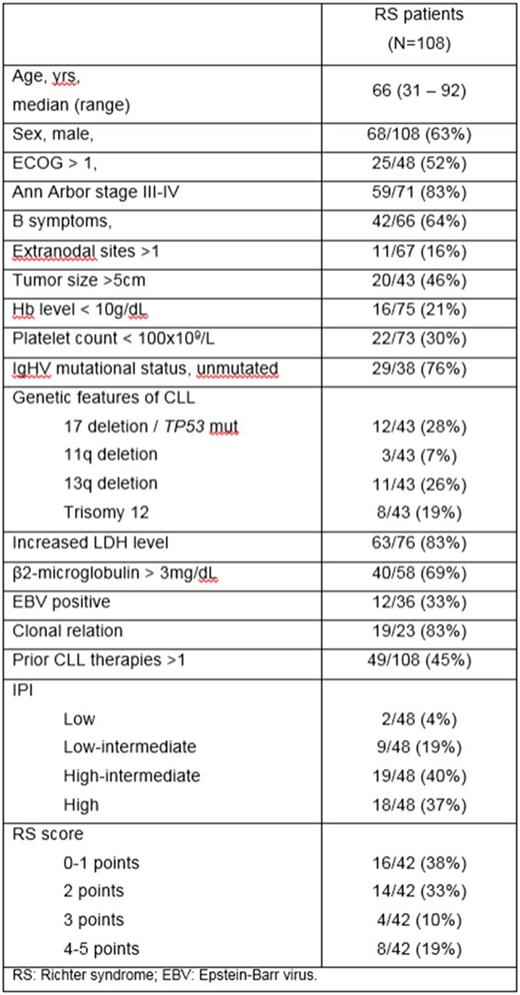
One hundred and twenty-three pts with transformation of CLL to high-grade lymphoma were identified, including 108 classical DLBCL RS, 14 pts with Hodgkin lymphoma (HL), and one case of plasmablastic lymphoma. Median age of the whole series was 66 years (range, 31 to 92 years), and 64% were men. For the DLBCL RS, median time from the diagnosis of CLL to RS development was of 4.7 years (range, 0-24.8 years, with 9 simultaneous diagnosis of CLL and RS), and the median number of prior therapies for CLL/SLL was 1 (range, 0-7). The main clinical characteristics of the pts are shown in table 1. EBV was detected in 12/36 (33%) cases with classical RS and in 9/11 (82%) of patients with HL variant. According to COO the majority of RS tested (20 of 24, 83%) had a non-germinal center phenotype, whereas 19/23 (83%) cases were clonally related to the preceding CLL. Twelve out of 43 pts had 17p deletion or mutation of TP53 in the CLL clone at the time of RS diagnosis.
Among the 108 patients with DLBCL RS, data on the treatment for RS was available in 99 patients. Twenty-one patients did not received treatment due to frailty, comorbidities, or poor performance status. Of the 78 patients that were treated for RS, 46 patients received chemoimmunotherapy, mainly R-CHOP (n=44), 29 patients chemotherapy (most commonly CHOP and purine analogue-based therapy), and others (n=3). The overall response rate for the 99 treated patients was 44% (chemotherapy, 24%; chemoimmunotherapy, 60%; P=0.003). A total of 13 patients underwent stem-cell transplantation (SCT), allogeneic SCT (n=7) or autologous SCT (n=6).
After a median follow-up for surviving patients of 36 months (range 0.8-160 months), median overall survival (OS) from the time of transformation of CLL to high grade lymphoma was 7.3 months for the entire cohort, 5.7 months for DLBCL RS patients, and 30.8 months for patients with HL variant (P=0.07). Factors associated with shorter OS in the DLBCL RS series included, ECOG PS >1, Hb level < 10 g/dL, platelet count < 100x109/L, LDH > 1.5 UNL, β2-microglobulin > 3mg/dL, prior CLL therapies >1, p53 positive determined by IHQ, and 17p deletion or mutation of TP53 determined in the CLL clone. RS score was also predictive for OS (median OS of 59.5 months [0-1 points], 5.4 months [2 points], 2 months [3 points], and 3 months [4-5 points], p= 0.006); on the contrary, IPI was not discriminative for OS in this series. In addition, RS clonally related presented a median OS of 5.9 months in comparison with 74.8 months for the clonally unrelated RS (p= 0.071). Finally, patients treated with chemoimmunotherapy combinations presented a median OS of 35.4 months, and the 13 patients that could consolidated the response obtained with SCT (autologous or allogeneic SCT) obtained a median OS of 59.5 months.
Although clinical outcome of patients with RS is generally poor, chemoimmunotherapy combinations are effective and obtain durable responses in a proportion of patients. RS score and TP53 disruption are predictive of survival. Finally, pts attaining a response should be considered for SCT. Further clinical development with novel promising treatment options is warranted.
Delgado: Abbvie, Jansen, Gilead, Roche: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.